Rank the bonds below in order of relative polarity – Embarking on an exploration of bond polarity, this discourse delves into the concept of ranking bonds based on their relative polarity. Understanding the polarity of bonds is crucial in chemistry, as it governs various molecular properties and influences chemical reactivity.
This article aims to provide a comprehensive overview of bond polarity, explaining the criteria for ranking bonds and presenting a detailed table for easy reference. Moreover, it explores the practical applications of bond polarity in chemistry and industry, highlighting its significance in shaping molecular behavior and driving chemical processes.
Polarity of Bonds
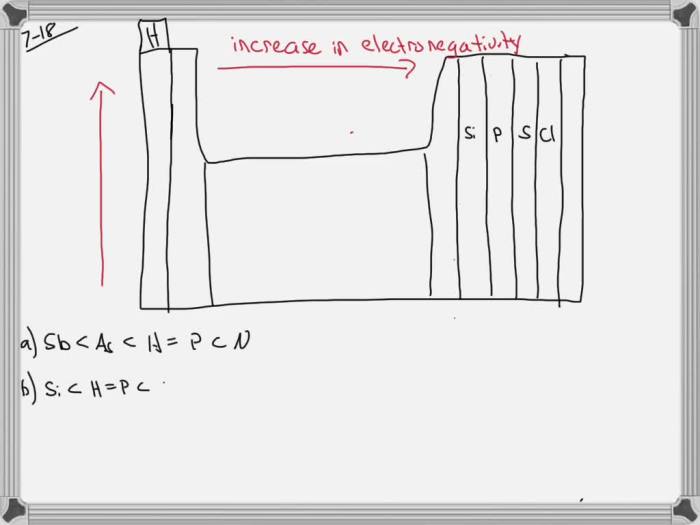
Bond polarity refers to the uneven distribution of electrons in a chemical bond. It arises when one atom in the bond has a greater electronegativity than the other, meaning it has a stronger attraction for electrons. The more electronegative atom will attract electrons towards itself, creating a partial negative charge on that atom and a partial positive charge on the less electronegative atom.
Polar bonds are formed between atoms with a significant difference in electronegativity, while nonpolar bonds are formed between atoms with similar electronegativity. For example, the bond between hydrogen and chlorine is polar because chlorine is more electronegative than hydrogen, while the bond between two hydrogen atoms is nonpolar because they have the same electronegativity.
Factors Affecting Bond Polarity, Rank the bonds below in order of relative polarity
- Electronegativity difference:The greater the difference in electronegativity between the bonded atoms, the more polar the bond.
- Atomic size:Larger atoms tend to be less electronegative than smaller atoms, leading to less polar bonds.
- Bond length:Shorter bonds tend to be more polar than longer bonds because the electrons are held more tightly in shorter bonds.
Ranking Bonds by Polarity
Bonds can be ranked by polarity based on the electronegativity difference between the bonded atoms. The greater the electronegativity difference, the more polar the bond.
| Bond Type | Electronegativity Difference | Dipole Moment (D) | Polarity |
|---|---|---|---|
| H-F | 1.9 | 1.82 | Polar |
| H-Cl | 0.9 | 1.03 | Polar |
| H-Br | 0.7 | 0.79 | Polar |
| H-I | 0.5 | 0.44 | Polar |
| C-H | 0.4 | 0.4 | Slightly Polar |
| C-C | 0 | 0 | Nonpolar |
Examples of Bond Polarity: Rank The Bonds Below In Order Of Relative Polarity
- H-F:Polar bond due to high electronegativity difference (1.9).
- C-H:Slightly polar bond due to a small electronegativity difference (0.4).
- C-C:Nonpolar bond due to no electronegativity difference (0).
Applications of Bond Polarity
Bond polarity has numerous applications in chemistry, including:
- Solubility:Polar molecules tend to be more soluble in polar solvents, while nonpolar molecules tend to be more soluble in nonpolar solvents.
- Reactivity:Polar bonds are more reactive than nonpolar bonds because the partial charges make them more susceptible to attack by other molecules.
- Industrial processes:Bond polarity is used in various industrial processes, such as the production of plastics, pharmaceuticals, and fuels.
Questions and Answers
What is bond polarity?
Bond polarity refers to the uneven distribution of electrons in a covalent bond, resulting in a partial positive charge on one atom and a partial negative charge on the other.
How is bond polarity determined?
Bond polarity is determined by the electronegativity difference between the bonded atoms. Electronegativity measures an atom’s ability to attract electrons towards itself.
What are the applications of bond polarity?
Bond polarity plays a crucial role in determining molecular properties such as solubility, reactivity, and intermolecular forces. It also finds applications in fields such as drug design, materials science, and industrial processes.